Curriculum - Ischemic Injury and Infarction
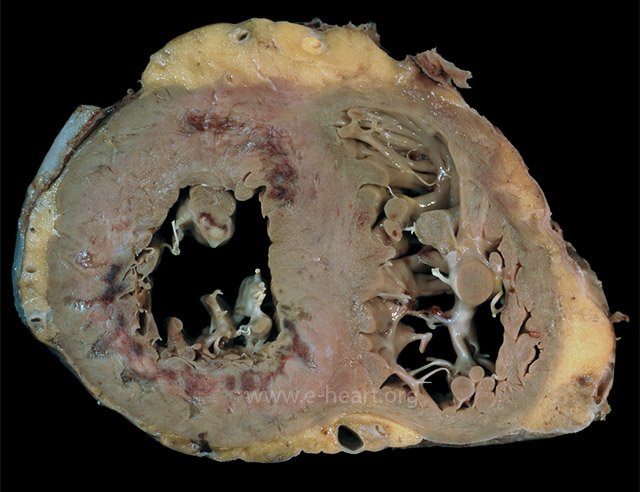 Cross section of the ventricles showing subendocardial infarcts in the anterior and posterolateral walls that extend into the septum. The infarcts have a gelatinous texture and the red areas represent granulation tissue> Bordering the infarcts there are subtle areas of white gray discoloration which represent areas of early scar formation. Also note the infarct in the anterolateral papillary muscle.
Cross section of the ventricles showing subendocardial infarcts in the anterior and posterolateral walls that extend into the septum. The infarcts have a gelatinous texture and the red areas represent granulation tissue> Bordering the infarcts there are subtle areas of white gray discoloration which represent areas of early scar formation. Also note the infarct in the anterolateral papillary muscle.
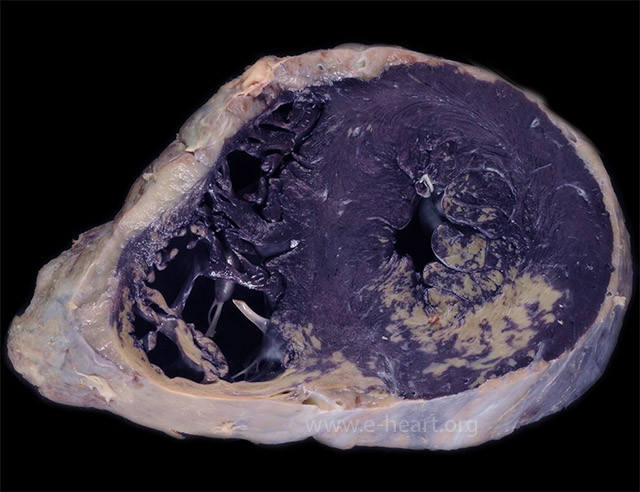 Cross section of the ventricles fixed in formalin after incubation in nitroblue tetrazolium chloride shows distinct infarcts (lack of blue/purple staining) in the posterior wall of the left and right ventricles. This stain accurately detects infarcts less than 4 hours in evolution, before any reliable histologic finding can be seen.
Cross section of the ventricles fixed in formalin after incubation in nitroblue tetrazolium chloride shows distinct infarcts (lack of blue/purple staining) in the posterior wall of the left and right ventricles. This stain accurately detects infarcts less than 4 hours in evolution, before any reliable histologic finding can be seen.
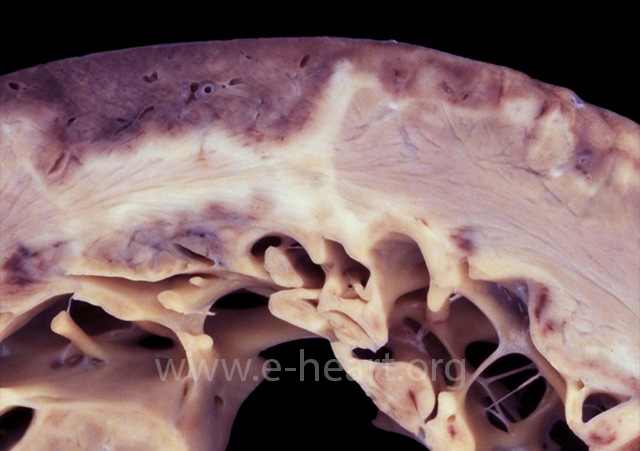 The myocardium shows a yellow demarcation between the viable subepicardial myocardium and infarcted subendocardial myocardium on the left. The infarct becomes transmural on the right side of the field. The yellow border represents the zone of maximal infiltration of neutrophils at 2-3 days
The myocardium shows a yellow demarcation between the viable subepicardial myocardium and infarcted subendocardial myocardium on the left. The infarct becomes transmural on the right side of the field. The yellow border represents the zone of maximal infiltration of neutrophils at 2-3 days
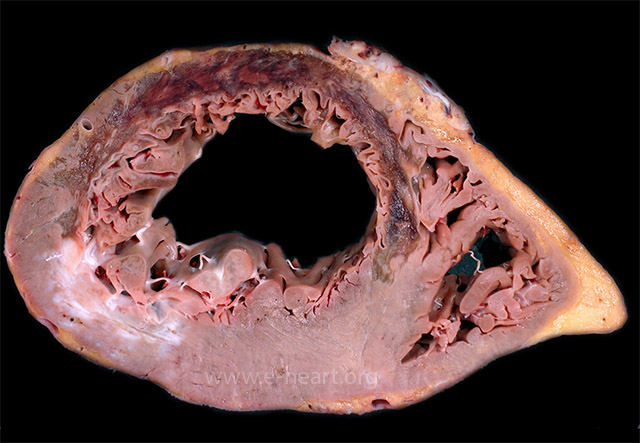 An extensive transmural anteroseptal left ventricular infarct shows thinning of the myocardium with gelatinous change consistent with early scar formation between 7 to 14 days. Islands of necrotic myocardium may persist in large infarcts as seen in the anterior wall in this case. A healed infarct with white scar is present in the posterolateral wall.
An extensive transmural anteroseptal left ventricular infarct shows thinning of the myocardium with gelatinous change consistent with early scar formation between 7 to 14 days. Islands of necrotic myocardium may persist in large infarcts as seen in the anterior wall in this case. A healed infarct with white scar is present in the posterolateral wall.
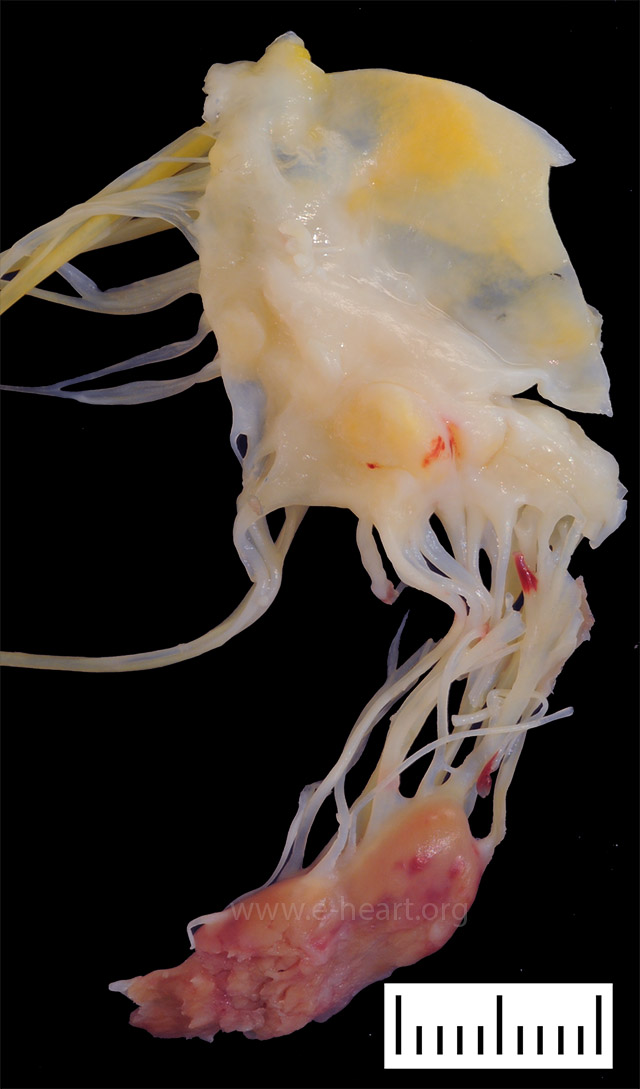 Surgical specimen showing a segment of mitral valve and a ruptured papillary muscle secondary to myocardial infarction. Note the pale myocardium with hemorrhages and the ragged edges of the papillary muscle head.
Surgical specimen showing a segment of mitral valve and a ruptured papillary muscle secondary to myocardial infarction. Note the pale myocardium with hemorrhages and the ragged edges of the papillary muscle head.
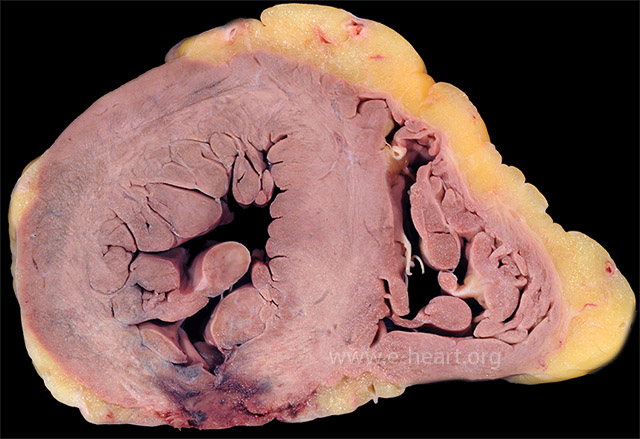 Acute transmural infarction in the posterior wall evolved into a rupture site. The image shows a serpiginous hemorrhagic path of the blood dissecting through the necrotic myocardium.
Acute transmural infarction in the posterior wall evolved into a rupture site. The image shows a serpiginous hemorrhagic path of the blood dissecting through the necrotic myocardium.
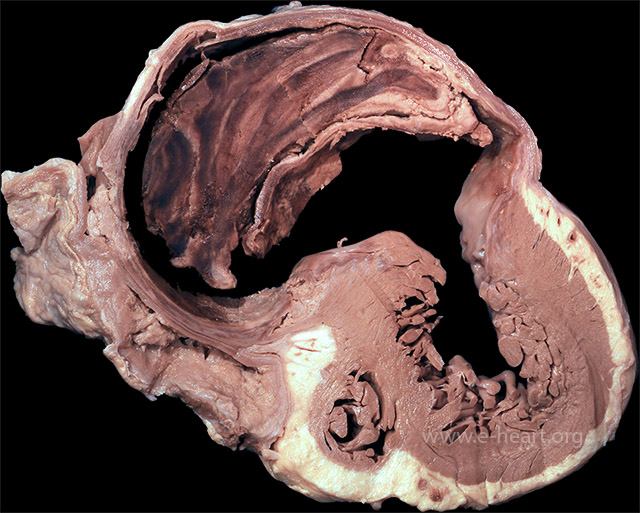 A pseudoaneurysm with laminated thrombus is shown surrounded by fibrous tissue and pericardium. A pseudoaneurysm results from a contained rupture of the ventricular wall and communicates with the ventricular cavity through a narrow neck. In comparison, a true ventricular aneurysm results from dilatation of the scarred myocardium.
A pseudoaneurysm with laminated thrombus is shown surrounded by fibrous tissue and pericardium. A pseudoaneurysm results from a contained rupture of the ventricular wall and communicates with the ventricular cavity through a narrow neck. In comparison, a true ventricular aneurysm results from dilatation of the scarred myocardium.
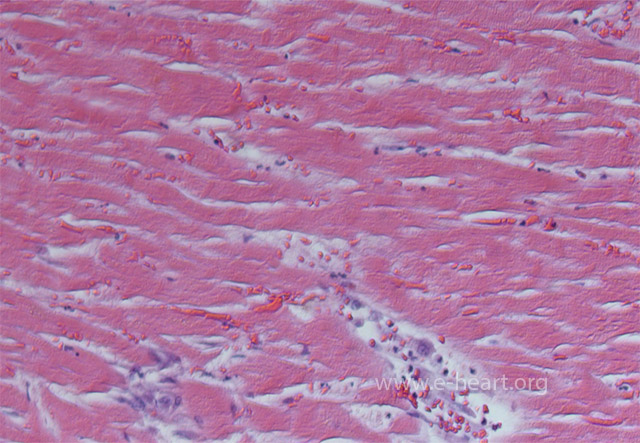 Coagulation necrosis of the myocardium showing hypereosinophilic sarcoplasm of the myocytes with indistinct or frankly blurred striations and loss of nuclei.
Coagulation necrosis of the myocardium showing hypereosinophilic sarcoplasm of the myocytes with indistinct or frankly blurred striations and loss of nuclei.
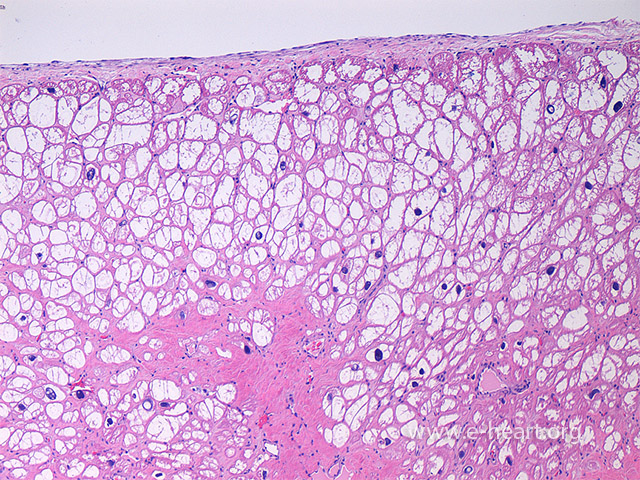 Colliquative myocytolysis showing large vacuolated sarcoplasm of myocytes due to hydropic change. It usually occurs in subendocardial location. It may also be seen in areas of “hibernating” myocardium in chronic ischemic injury.
Colliquative myocytolysis showing large vacuolated sarcoplasm of myocytes due to hydropic change. It usually occurs in subendocardial location. It may also be seen in areas of “hibernating” myocardium in chronic ischemic injury.
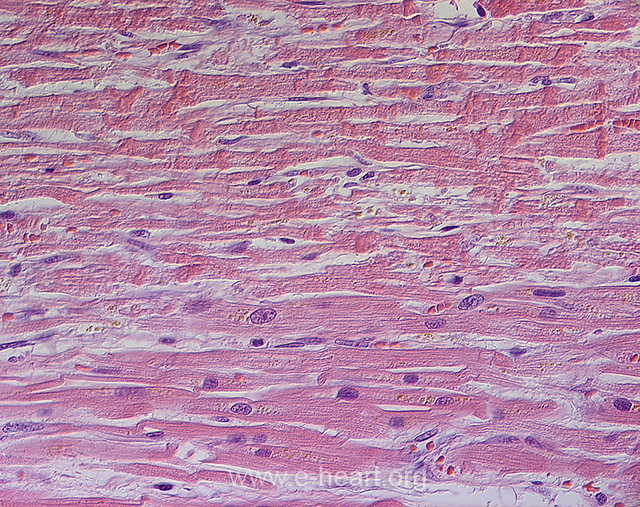 Contraction band necrosis showing transverse hypereosinophilic bands alternating with pale granular spaces along the length of the myocytes. The transverse bands result from overlapping of hypercontracted sarcomeres. For comparison the myocytes in the lower portion of the field do not show contraction band necrosis.
Contraction band necrosis showing transverse hypereosinophilic bands alternating with pale granular spaces along the length of the myocytes. The transverse bands result from overlapping of hypercontracted sarcomeres. For comparison the myocytes in the lower portion of the field do not show contraction band necrosis.
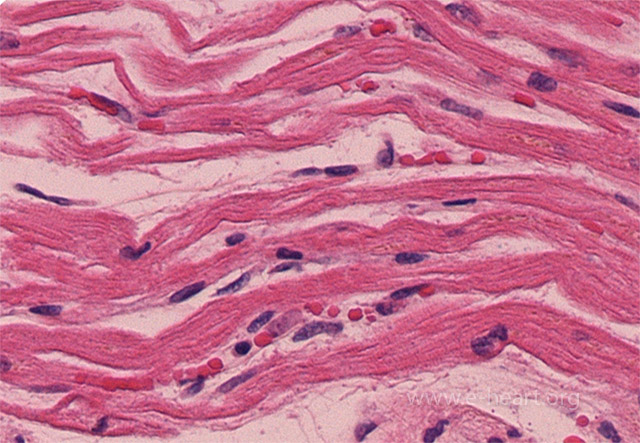 An early morphologic change in acute myocardial infarction is the appearance of wavy and thinned fibers. Note the capillary congestion in these areas, lack of polymorphonuclear infiltration and presence of hypereosinophilic fibers in the myocytes.
An early morphologic change in acute myocardial infarction is the appearance of wavy and thinned fibers. Note the capillary congestion in these areas, lack of polymorphonuclear infiltration and presence of hypereosinophilic fibers in the myocytes.
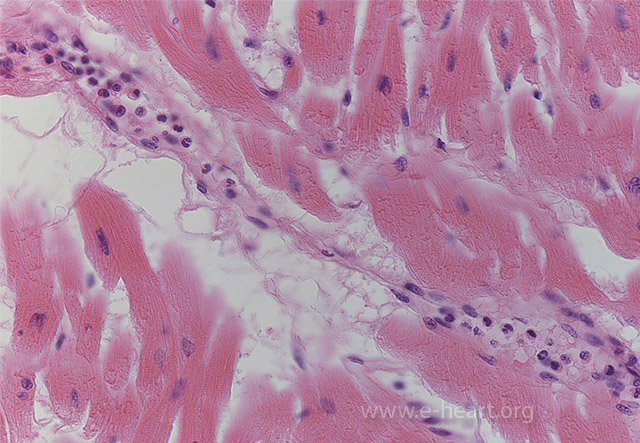 Margination of polymorphonuclear leukocytes is one of the earliest unambiguous changes in myocardial infarcts. It is seen as early as 4 to 6 hours postinfarction.
Margination of polymorphonuclear leukocytes is one of the earliest unambiguous changes in myocardial infarcts. It is seen as early as 4 to 6 hours postinfarction.
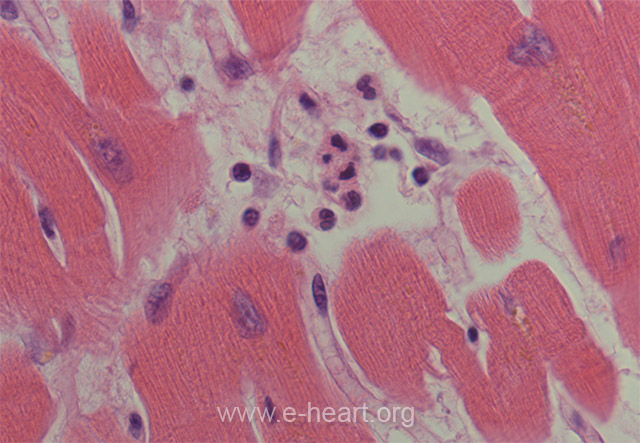 Once the polymorphonuclear leukocytes marginate inside capillaries near the infarcted area, they begin to diapedese into the extracellular space and infiltrate the surrounding myocardium. This change usually starts at around 6-8 hours and increases with time as more polymorphonuclear leukocytes are chemoattracted to the infarcted myocardium.
Once the polymorphonuclear leukocytes marginate inside capillaries near the infarcted area, they begin to diapedese into the extracellular space and infiltrate the surrounding myocardium. This change usually starts at around 6-8 hours and increases with time as more polymorphonuclear leukocytes are chemoattracted to the infarcted myocardium.
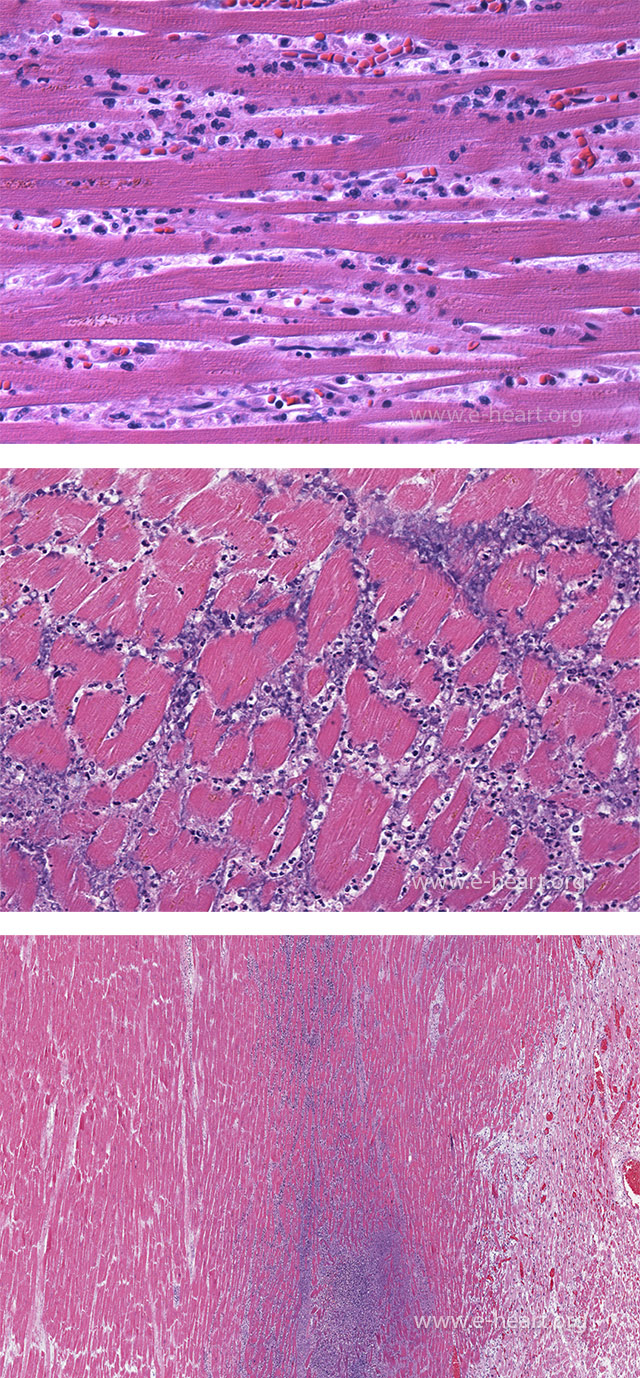 The top panel shows frank infiltration of the interstitium by further diapedesis of polymorphonuclear leukocytes. The myocardium shows coagulation necrosis. This amount of polymorphonuclear leukocyte infiltration occurs at around 24 hour of evolution of the infarct. The nuclei of the myocytes are not staining but striations can still be identified in the sarcoplasm. The middle panel shows extensive karyorrhexis of the polymorphonuclear leukocytes, which imparts a “dusty” basophilic appearance and is observed at 3-4 days postinfarct. The lower panel shows a zone of basophilia representing polymorphonuclear cells undergoing karyorrhexis between the zone of coagulative necrosis on the left and viable myocardium on the right.
The top panel shows frank infiltration of the interstitium by further diapedesis of polymorphonuclear leukocytes. The myocardium shows coagulation necrosis. This amount of polymorphonuclear leukocyte infiltration occurs at around 24 hour of evolution of the infarct. The nuclei of the myocytes are not staining but striations can still be identified in the sarcoplasm. The middle panel shows extensive karyorrhexis of the polymorphonuclear leukocytes, which imparts a “dusty” basophilic appearance and is observed at 3-4 days postinfarct. The lower panel shows a zone of basophilia representing polymorphonuclear cells undergoing karyorrhexis between the zone of coagulative necrosis on the left and viable myocardium on the right.
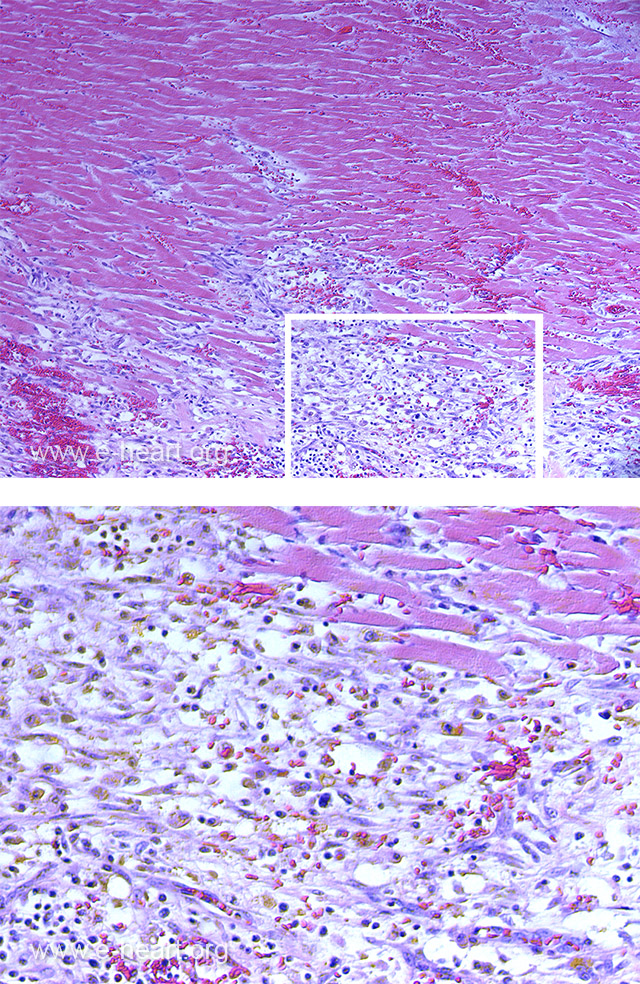 Most of the myocytes show hypereosinophilia of the sarcoplasm with coagulation necrosis and loss of nuclei. Other myocytes appear pale with lysis of myofibrils. Note the extensive infiltration by macrophages, most of which contain yellow brown pigment (hemosiderin and/or lipofucsin from engulfing myocyte debris). This infarct is about 7 days in in evolution.
Most of the myocytes show hypereosinophilia of the sarcoplasm with coagulation necrosis and loss of nuclei. Other myocytes appear pale with lysis of myofibrils. Note the extensive infiltration by macrophages, most of which contain yellow brown pigment (hemosiderin and/or lipofucsin from engulfing myocyte debris). This infarct is about 7 days in in evolution.
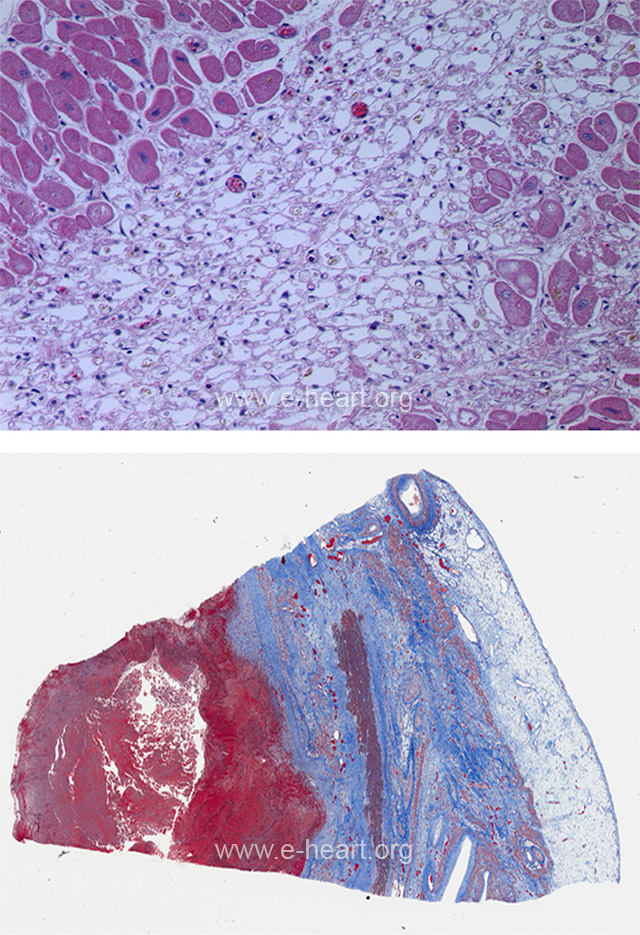 The disappearance of myofibrils leaves empty spaces bounded by the sarcolemma resulting in an alveolar pattern with scattered macrophages. This pattern is usually seen in small areas of infarction or in the outer zone of a large infarct. The lower image shows a healing transmural infarct with pale and dark blue zones of collagen deposition. Note the mural thrombus with the red fibrin in a trichrome stain.
The disappearance of myofibrils leaves empty spaces bounded by the sarcolemma resulting in an alveolar pattern with scattered macrophages. This pattern is usually seen in small areas of infarction or in the outer zone of a large infarct. The lower image shows a healing transmural infarct with pale and dark blue zones of collagen deposition. Note the mural thrombus with the red fibrin in a trichrome stain.
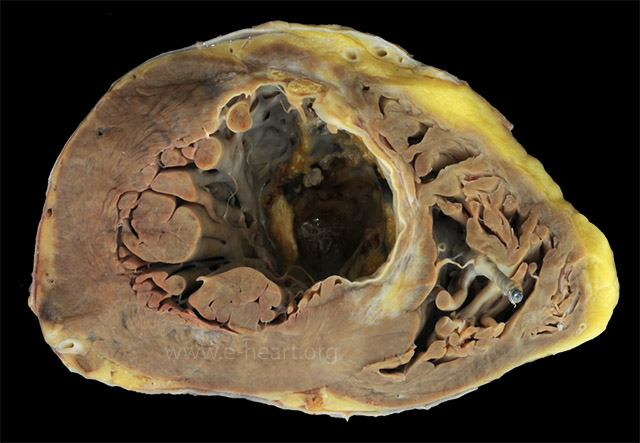 A healed transmural infarct of the anterior septum and a small portion of the anterior left ventricular wall appears as dense white scar with focal calcification. There is an apical thrombus. Note the fine areas of grey white fibrosis in the non-infarcted myocardium. The right ventricle shows a segment of a pacing lead with a fibrous tissue cuff surrounding it.
A healed transmural infarct of the anterior septum and a small portion of the anterior left ventricular wall appears as dense white scar with focal calcification. There is an apical thrombus. Note the fine areas of grey white fibrosis in the non-infarcted myocardium. The right ventricle shows a segment of a pacing lead with a fibrous tissue cuff surrounding it.

